Dr. Kiranmai Nayani
Research Group
Organic Synthesis & Process Chemistry







Total synthesis of bioactive molecules
Total synthesis of bioactive moleculesThe total synthesis of complex natural products remains among the most fascinating and dynamic research areas. In our group, we focus on advanced chemical transformations for molecule synthesis. Whenever feasible, we try to avoid protective groups and utilize green solvents in order to accomplish efficient, cost-effective, and sustainable synthesis.

Synthesis of bioactive peptides
Peptides have gained increased interest as therapeutics in recent years. More than 60 peptide drugs have reached the market for the benefit of patients and several hundreds of novel therapeutic peptides are in preclinical and clinical development. Currently, we are focusing on the efficient and cost-effective green synthesis of peptides.
Late-stage functionalization (LSF) of peptides
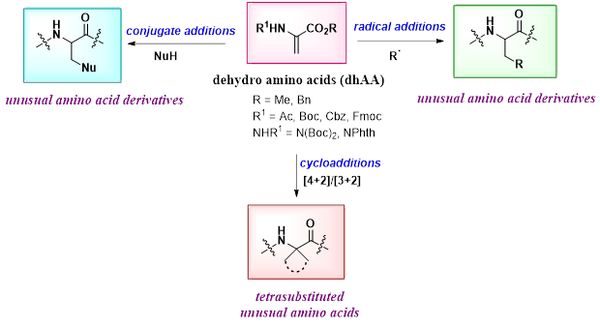
Peptides have emerged as powerful building blocks in drug discovery and chemical biology. Along with the gain in interest in peptides as therapeutics, and molecular probes, enormous demand has been noticed for late-stage functionalization (LSF) of peptides to make them less susceptible to enzymatic hydrolysis and to provide a better fit in the protein cavities. In this regard, site-selective functionalization of peptides has received great attention to achieve structural diversity quickly for improved pharmacokinetics and biological activities. Unnatural amino acids generated through LSF have become key structural motifs in peptide-based drugs, which prompted our group to explore a variety of chemoselective transformations to achieve structural diversity in peptides.
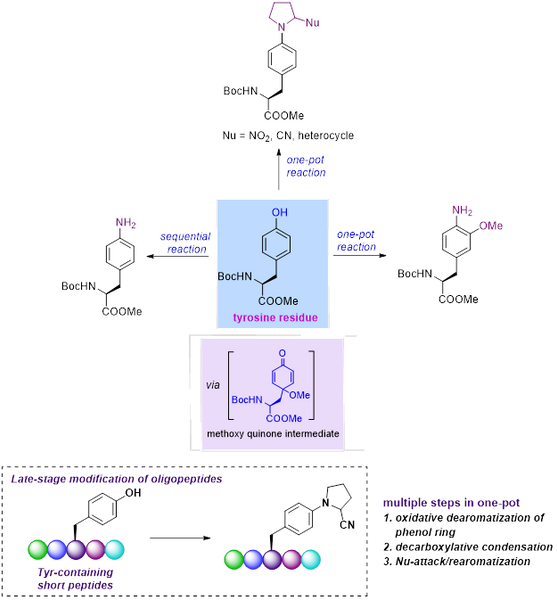
Tyrosine modification in short peptides
Diversity-oriented approach to unusual amino acid derivatives